An introduction to
CHEMICAL FOSSIL PREPARATION

What is chemical preparation?
Chemical preparation is the use of chemical substances to either dissolve or disintegrate the rock around the fossil, to chemically alter the fossil (e.g. to treat pyrite decay), or to dissolve the fossil from the rock to allow for a cast to be made.
Fossil preparators can use acids, alkalis and even surfactants to reveal fossils where mechanical preparation techniques may not be as or at all effective. Which chemical you might use depends entirely on the composition of the rock and the composition of the fossil. If they are of the same composition, chemical preparation is not an option.
Perhaps the most well-known method is acid preparation, using either acetic or formic acid to dissolve calcareous rocks that contain fossilised vertebrates. Sounds simple, right? Dunk the rock in some acid and come back later and you've got a beautifully exposed fossil? Wrong. Chemical preparation requires a lot of skill to get it right. That said, virtually all fossil preparators are self-taught, and they all started somewhere. Reading this article will get you well on your way to understanding how it works, why it works, where it can go wrong and why, and of course how to keep you and your fossil safe during the process. Done right, chemical prep using acids or alkalis can produce absolutely stunning results.
Alkalis can also be used, such as Potassium Hydroxide (KOH), as can surfactants like Rewoquat. Different techniques are used depending on the rock type and the mineral composition of the fossil. We will cover acid preparation, KOH preparation and Rewoquat preparation in more depth.
Treatment of pyrite decay is a chemical alteration process and therefore comes under 'chemical preparation', but we have a whole separate article on that linked here. Here we will discuss other chemical techniques.

We have two main rules for chemical preparation in the home workshop:
1. YOU and your safety must be your number one priority. No fossil is worth your health. These chemicals can be extremely dangerous if handled improperly or without suitable PPE. Always read the safety data sheet and follow the instructions for use, storage, handling and understand what steps to take in an emergency or in case of spillage. Refresh your knowledge every time you use these chemicals - it's amazing how quickly you can forget. Access to fresh, running water, an eye wash, as well as any further materials advised for specific chemicals is a must.
2. If you haven't used a chemical for prep before, or not used it on a fossil from a certain locality, practice first on a scrap or inferior piece.
Basically, thou shalt not do damage to thyself.
Followed by thou shalt not damage thine fossil.
WHICH CHEMICAL TECHNIQUE DO I USE AND WHEN?
Chemical preparation relies entirely on a difference in chemical composition between the fossil and the rock. In theory, this allows you to preferentially remove the matrix, leaving the fossil behind. For example, chemical preparation is unsuitable for a calcite ammonite preserved in limestone as chemically, they are both CaCO₃ (carbonate), but could potentially be used for a calcium phosphate (bone) fossil in the same limestone. Depending on the matrix and the fossil within, there are a variety of chemical techniques available to the fossil preparator.
Our first question must be - when is chemical preparation an option? Chemical preparation is a possibility if the results produced are either unachievable via mechanical preparation means (air scribes, air abrasives, etc) or the results would be better than if prepared using mechanical means. One situation may be where the rock is considerably harder than the fossil, and so air abrasives cannot be used as they would damage the fossil before they remove the rock. If the fossil preservation and rock type are in your favour, chemical prep may be an option.
If you think chemical preparation is a potential method to use, you must then establish what type of fossil you have and how it is preserved. Vertebrates are preserved as calcium phosphate, but shelly invertebrates can have calcite/aragonite preservation (e.g. ammonites, bivalves, brachiopods etc.). But they can also have preservation that is not calcite, and potentially the same as the rock (e.g. many ammonites do not have calcite shells/infill preserved and instead have mud infill, or some chambers (especially the body chamber) with mud infill. Carbonised fossils like plants, pyrite fossils, and other minerals are not suitable for preparation using acid, potassium hydroxide or surfactants.
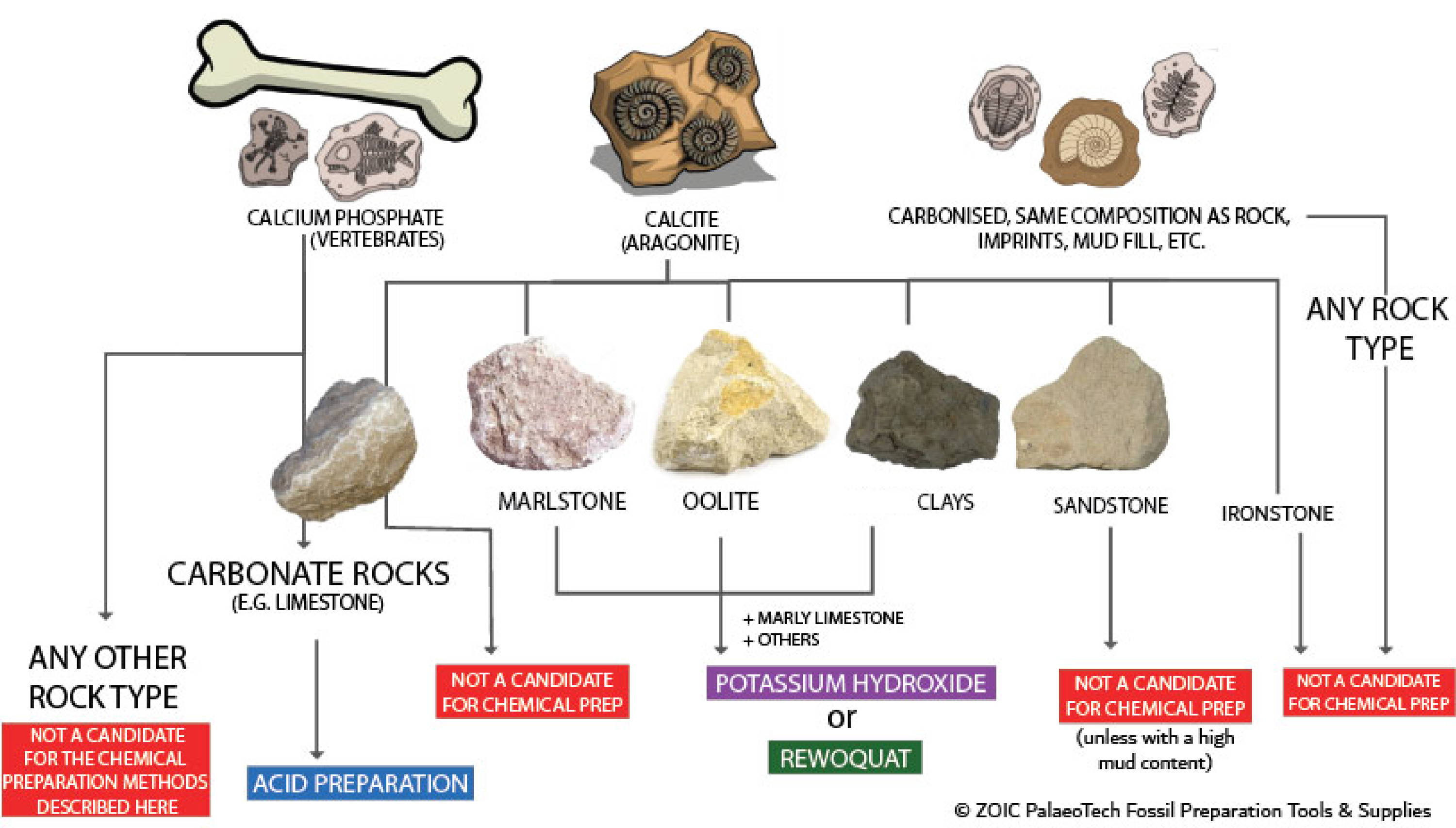
Not all fossils, even if they fit the above criteria, are good candidates for chemical prep. This chart is a simplified indication of what might work, and what really won't. Bear in mind that bones will be preserved as calcium phosphate, but when they are preserved in limestone they almost always have the pore spaces infilled with calcite which poses difficulties for acid prep. There are ways to get around this, but there are also some fossils from some localities which are almost impossible to acid prep successfully.
There are some techniques we won't go into in these articles, as either the chemicals are extremely difficult to use successfully or the preservational style is very rare, e.g. the Waller Method using thioglycollic acid on fossils preserved in ironstone. Hydrofluoric acid can be used to dissolve siliceous rocks (e.g. quartz, etc.) but success is usually slim.
If you aren't sure what type of preservation your fossil has, or the rock that it's in, do a bit of research on the locality you found your fossil and find out more about the geology. Perhaps ask some people who collect fossils there and they will hopefully be able to help you!
A quick chemistry recap - what you need to know before you start chemical prepping
Before we go too much further, we'll recap some basic chemistry that you might have forgotten since you left school - you might even remember thinking "I'll never use this". We just need to understand the very basics of the language used when reading our articles to make the most of them.
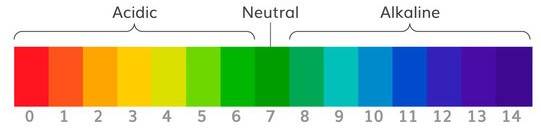
This colourful scale shows pH, a scale of acidity or alkalinity. Acids are 0-6 on the scale, 7 is neutral (e.g. water) and anything 8-14 is considered alkaline. If you add water to an acid, you will increase the pH, bringing it closer to neutral. If you add water to an alkali, you will decrease the pH, again bringing it closer to neutral. When we talk about 'neutralising', we mean just that - bringing a solution back to a neutral pH of 7.
Learn about the various Chemical Preparation Methods
Now we've introduced you to chemical prep, find out more about each individual method by clicking the links below. We provide examples and case studies in each article.
Learn more about each of the techniques by clicking the links below:
PLEASE READ THIS SECTION - IT'S IMPORTANT!
STAYING SAFE WHEN WORKING WITH CHEMICALS
We will do our BEST not to make this bit boring, as it's super important that you read this before trying out any of the techniques described below. People tend to be a bit more cavalier when we’re working at home, on our own time and on our own fossils, but health and safety regulations are in place in workplaces to protect you.
Some of these chemicals, just because they are available to purchase by the general public, does not mean they are not extremely dangerous. Research what the risks involved are, read the safety data sheets, and take all necessary precautions to reduce the chance of spillage, accident or injury. We are not liable in any way for any damages incurred to you, you property, or anyone around you. You are responsible for understanding and executing safe and competent hazardous chemicals handling.
Always refresh your knowledge of the safety data sheets before use, even if you think you can remember from last time. Know what to do in case of inhalation, ingestion, eye contact, skin contact or spillage. Be aware of how to store your chemicals safely as this may impact property insurance. Make sure to keep all chemicals out of reach of children and pets. Follow all specific instructions for storage as per the safety data sheet.
Always have access to fresh running water, a first aid kit, an eye wash station and a mobile phone or the means to get help in an emergency.
Always wear suitable (chemical-resistant) protective gloves, goggles (not just glasses - full goggles!) and protective clothing. If further PPE is recommended, this will be listed in the safety data sheet.
As a general rule for all fossil prep - don't inhale anything. That goes for most of what we do - rock dust, glues, acids, acetone, it's all bad for you. Wear a suitable respirator or FFP3/N99 grade dust mask (this grade filters out vapours too). Work in a well ventilated environment.
There's a bit of a misconception that acids are more dangerous than alkalis. Alkalis can be really horrible and do far more harm than many acids. The product that should be used with an abundance of caution here is Potassium Hydroxide. You can lose your eyesight with minimal eye contact. We don't want to frighten anyone, but equally it's so important to go into chemical prep armed with as much knowledge on how to protect yourself as possible.
SHARING OUR KNOWLEDGE
We are committed not only to making the best fossil preparation tools, but we also love to share our knowledge to help you prep your best. If you feel that we are missing something important from this article, or have any photographs you would be happy to share with us we would be delighted if you drop us an email! We love to see before and afters, learn new tricks and see what you've been up to! We can be contacted using the link below or on info@zoicpalaeotech.co.uk.
